Depus Quick Response shunt is designed to regulate and sustain the intra-ventricular pressure (IVP) of the patient via controlled drainage of the CSF (cerebrospinal fluid).
The shunt has two versions according to intention of use: ventriculoperitoneal and lumboperitoneal.
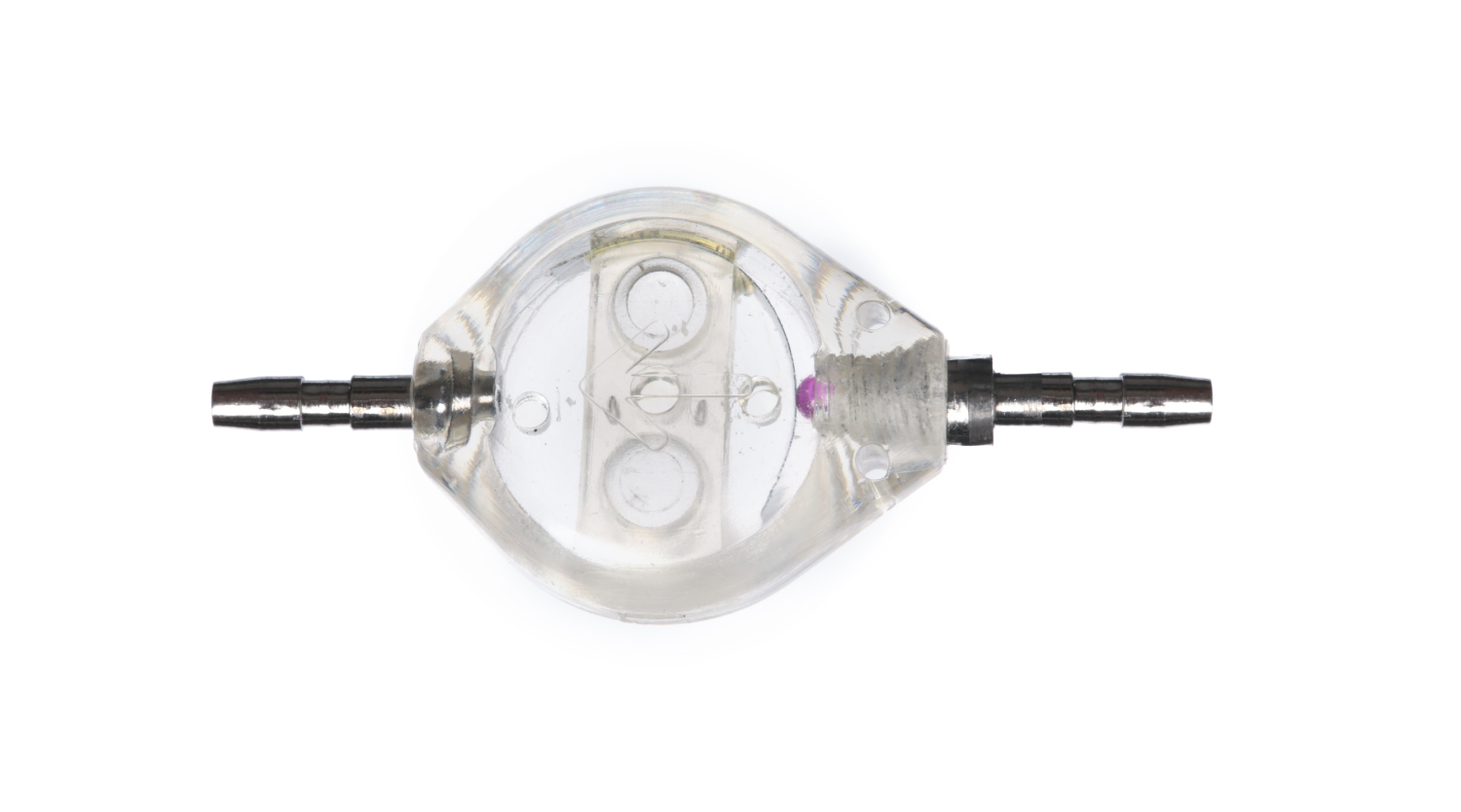
The mechanism within the shunt is triggered by positive ventricular pressure and the valve opens immediately. The ruby ball and the titanium spring valve are the essentials of this adjustable system that works on the theory of hydrodynamic leverage. The shunt body is designed to ave uniquely smaller dimensions than its competitors. Inner diameter of the valve is 10 mm, outer diameter is 13,5 mm and the length is 16 mm.
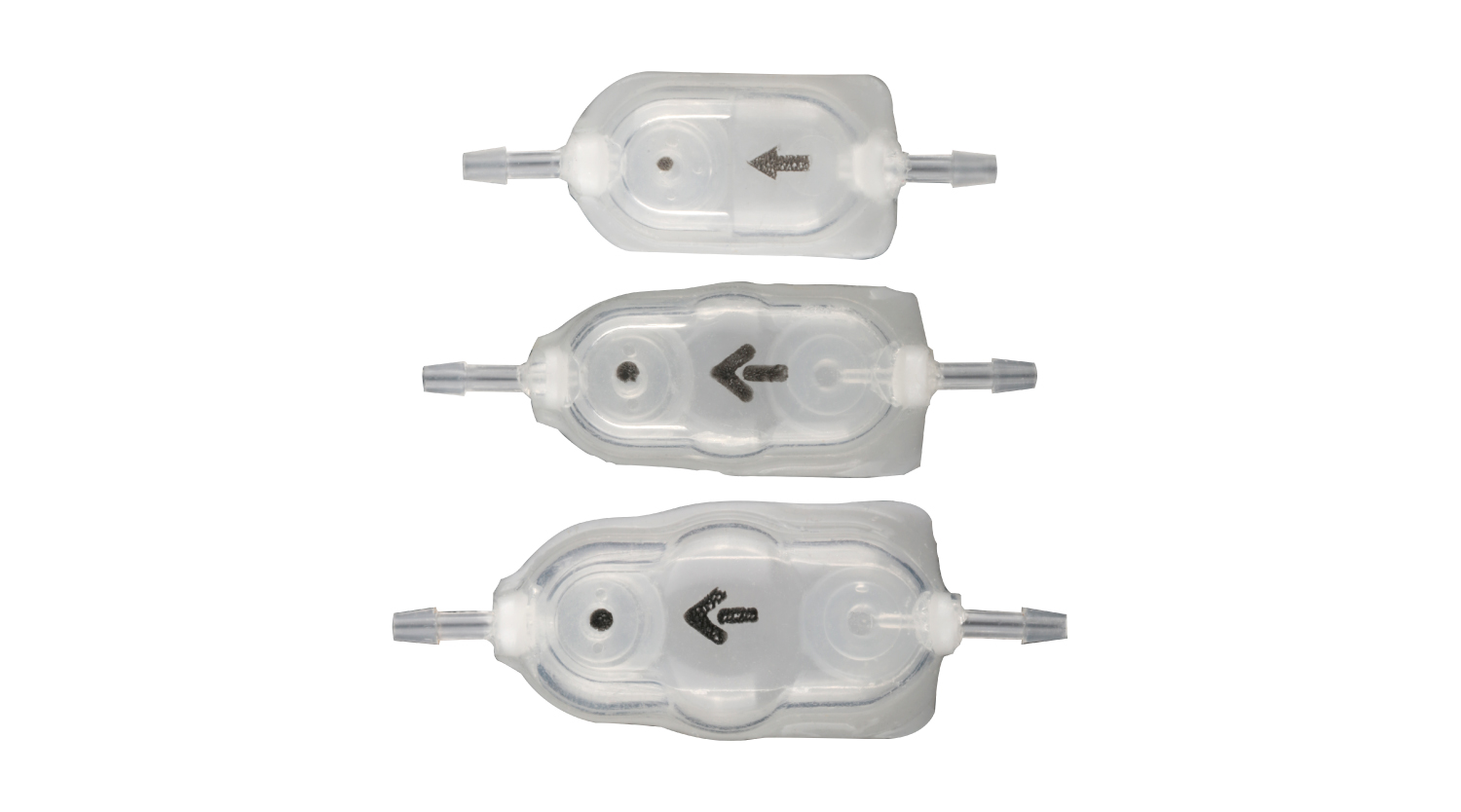
Depus Quick Response shunt is also designed with aninfection-preventing version which includes an antibiotic impregnated silicone cover.
This antibiotic impregnated body design of the Depus valve is unique in thecurrent shunt market.
Controlled release of the impregnated antibiotics from the silicone body for 28 days is also the ultimate method for infection prevention, which is one of the main problems of shunt surgery.
* Please see our catalog for details.
Depus Quick Response Shunts ( Standard Valve Body / Antibiotic Impregnated Silicone Body )
Polysulphone shunts are manufactured using radiopaque titanium, ruby ball and polysulphone (long term implantable) and are supplied sterile (ETO). The shunt pressure levels are marked with tantalum which allows MRI visibility.
Depus Quick Response Shunt Kits ( Standard / Semi Antibiotic Impregnated / Full Antibiotic Impregnated )
Standard Depus Quick Response Shunt Kits | Semi Antibiotic impregnated Depus Quick Response Shunt Kits | Full Antibiotic Impregnated Depus Quick Response Shunt Kits |
Polysulphone body design enhanced with titanium spring and ruby ball MRI and CT compatible design that does not contain metal parts Tantalum markings to indicate direction of flow | Major advantage compared to Standard Depus Quick Response shunts due to antibiotic impregnated catheters Wide spectrum of protection through joint impregnation of Clindamycin HCl and Rifampicin Prevention of bacteria colonization up to 28 days Low obstruction risk due to hydrophilic nature of catheters | Antibiotic impregnated catheters and antibiotic impregnated silicone cover on valve body Wide spectrum of protection through joint impregnation of Clindamycin HCl and Rifampicin Prevention of bacteria colonization up to 28 days Low obstruction risk due to hydrophilic nature of catheters and shunt body |